Embark on a journey into the realm of density with our meticulously crafted Density Practice Problem Worksheet Answers Key. This invaluable resource provides a comprehensive understanding of density, its applications, and the intricate factors that influence it. Prepare to delve into the fascinating world of matter and its properties.
Within this guide, you will encounter a wealth of knowledge, including the precise definition of density, its units of measurement, and captivating real-life examples that showcase its practical significance. Our team of experts has meticulously designed a series of practice problems of varying difficulty levels, empowering you to test your comprehension and solidify your understanding.
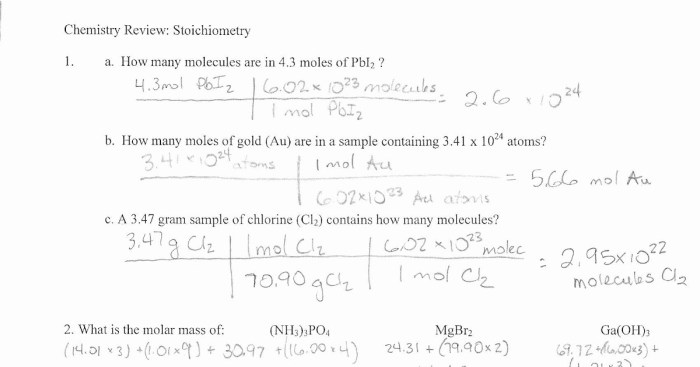
Density Practice Problems
Density is a fundamental property of matter that measures the mass of a substance per unit volume. It is an important concept in many scientific and engineering fields, and it has numerous practical applications.
Definition of Density, Density practice problem worksheet answers key
Density is defined as the mass of a substance divided by its volume. The SI unit of density is kilograms per cubic meter (kg/m 3), but it can also be expressed in grams per cubic centimeter (g/cm 3) or other units.
For example, the density of water is approximately 1 g/cm 3, which means that 1 cubic centimeter of water has a mass of 1 gram. The density of gold is approximately 19.3 g/cm 3, which means that 1 cubic centimeter of gold has a mass of 19.3 grams.
Calculating Density
The formula for calculating density is:
ρ = m/V
where:
- ρ is the density (in kg/m 3or g/cm 3)
- m is the mass (in kg or g)
- V is the volume (in m 3or cm 3)
To use this formula, simply divide the mass of the substance by its volume. For example, if you have a sample of water with a mass of 100 grams and a volume of 100 cubic centimeters, the density would be:
ρ = 100 g / 100 cm3= 1 g/cm 3
It is important to note that accurate measurements of mass and volume are essential for obtaining accurate density calculations.
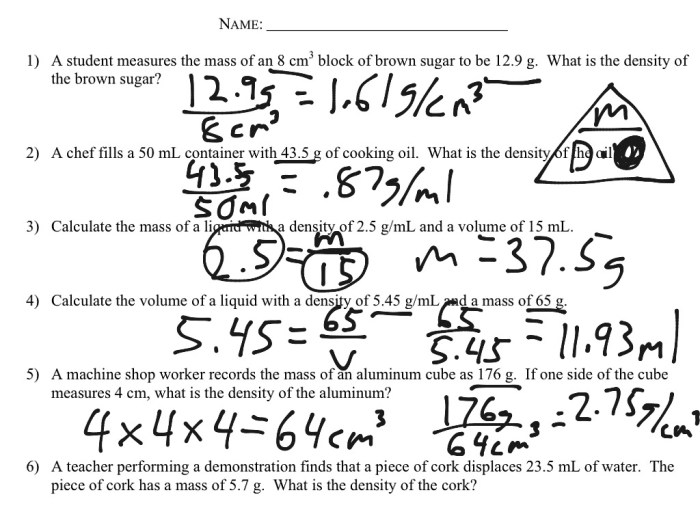
Density Practice Problems
| Problem | Solution |
|---|---|
| Calculate the density of a sample of metal with a mass of 50 grams and a volume of 10 cubic centimeters. | ρ = m/V = 50 g / 10 cm3 = 5 g/cm3 |
| A cube of wood has a side length of 10 centimeters. If the mass of the cube is 100 grams, what is its density? | The volume of the cube is V = a3 = 10 cm x 10 cm x 10 cm = 1000 cm3. Therefore, the density is ρ = m/V = 100 g / 1000 cm3 = 0.1 g/cm3 |
Applications of Density
Density has numerous practical applications in various fields, including:
- Science:Density is used to identify and classify substances, study the behavior of fluids, and understand the structure of matter.
- Engineering:Density is used to design and build structures, vehicles, and other products.
- Manufacturing:Density is used to control the quality of products and ensure they meet specifications.
Factors Affecting Density
The density of a substance can be affected by several factors, including:
- Temperature:As the temperature of a substance increases, its density typically decreases.
- Pressure:As the pressure on a substance increases, its density typically increases.
For example, the density of water decreases as its temperature increases, which is why hot water rises to the top of a container. The density of air increases as its pressure increases, which is why air is denser at sea level than at high altitudes.
FAQ Corner: Density Practice Problem Worksheet Answers Key
What is the formula for calculating density?
Density = Mass / Volume
How can I improve my accuracy in density calculations?
Ensure precise measurements of mass and volume using calibrated instruments and proper techniques.
What are some practical applications of density?
Density plays a crucial role in fields such as engineering, manufacturing, and quality control to determine the composition, purity, and authenticity of materials.